Core electron bonding may not always require extreme pressure, study finds
- Oct 1, 2025
- 4 min read

You probably learned in high school chemistry class that core electrons don’t participate in chemical bonding.
They’re thought to be too deep inside an atom and close to the nucleus to meaningfully interact with the electrons of other atoms, leaving the outer valence electrons to get all the chemical bonding glory in textbooks.
The actual science is more complicated, as some elements’ core electrons are theorized to activate when squeezed hard enough, like at the pressure levels found deep inside the Earth.
University at Buffalo researchers are now theorizing that core electron bonding may not always require as much pressure as previously thought. In fact, for some elements, it may only take the atmospheric pressure you’re experiencing right now on the Earth’s surface.
The researchers’ quantum chemical calculations, described in a study published in this month’s issue of the Journal of the American Chemical Society, revealed insights on the semicore electrons of alkali metals, a group of highly reactive elements located on the first row of the periodic table. The findings include:
Alkali metals’ semicore electrons can participate in bonding under just a few gigapascals of pressure — levels found in the deep crust and upper mantle but far lower than the hundreds of gigapascals once thought to be required for core election bonding.
In the case of one alkali metal, cesium, they can participate in bonding even at ambient pressure levels, roughly a million times lower than the pressures deep inside the Earth.
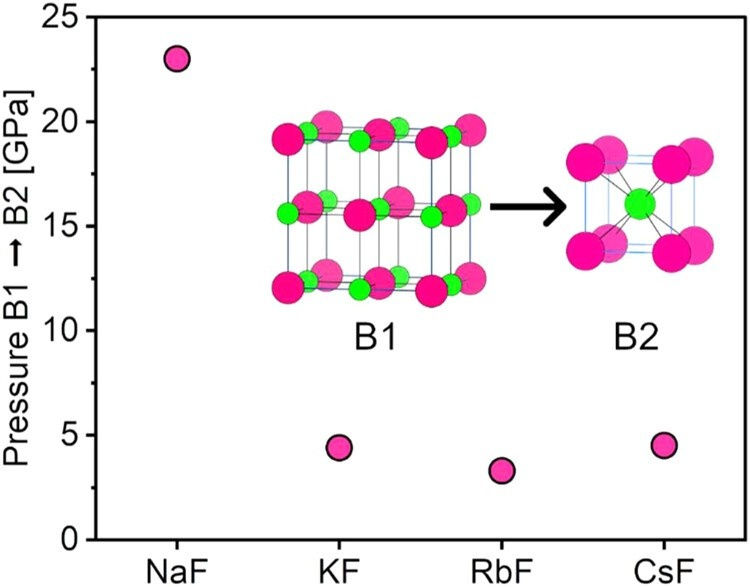
They play a pivotal role in what’s known as the B1–B2 transition, in which pressure causes a compound’s atomic crystal structure to rearrange from an octahedral shape (as seen in sodium chloride) to a more cubic shape (as seen in cesium chloride).
“These findings go against certain chemistry paradigms, challenging the traditional notions of core electrons,” says Eva Zurek, PhD, SUNY Distinguished Professor in the UB Department of Chemistry, who was the co-corresponding author of the study. “This kind of work could change our understanding of how elements change under the intense pressures inside planets and even how planets form and evolve.”
The work was supported by the Center for Matter at Atomic Pressure, a National Science Foundation center led by the University of Rochester.
Quantum chemical calculations reveal electrons’ true role
Describing the behavior of electrons isn’t easy. The Schrödinger equation does this but is practically unsolvable due to the enormous number of interactions between electrons.
That’s where quantum chemical calculations come in. Using mathematical approximations, various models have been developed that make the equation solvable and reveal the electronic structure of complex materials.
Zurek and the study’s other co-corresponding author, Stefano Racioppi, PhD, relied on models powered by UB’s Center for Computational Research to study the semicore electrons of alkali metals. They focused on what happens when these metals bond with fluorine and undergo the B1–B2 transition under pressure.
“We found that the metals’ semicore electrons were participating in bonding and that this bonding helped both drive and stabilize the B2 cubic structure,” says Racioppi, a former postdoctoral researcher in Zurek’s lab who is now an associate researcher at the University of Cambridge in the United Kingdom. “From this, we deduced that semicore electron bonding requires only a few gigapascals of pressure — far less than the hundreds once predicted.”
The B2 structure is the arrangement that cesium chloride adopts at ambient pressure — that is, under practically no pressure at all. By examining cesium chloride, Zurek and Racioppi calculated that cesium’s semicore electrons participate in bonding at ambient pressure.
“This suggests that the activation of semicore electrons might not be as rare as we once thought,” Zurek says. “It may be happening right here on Earth’s surface, without the need for extreme conditions.”
Rethinking how planets evolve
These kinds of insights improve the fundamental data that scientists use to model what happens to elements deep inside Earth and Earth-like planets.
“If electrons undergo different bonding than previously thought, it could change our understanding of a planet’s radius, plate tectonics and magnetic field generation, all of which influence whether a planet can sustain life,” Zurek says.
The study suggests some potential next steps for future experiments, such as using X-ray diffraction to better characterize the atomic structure of alkali metals and thus the role of their semicore electrons in chemical bonding.
“This is hopefully not just a theoretical study, but also a roadmap for experimentalists to prove or disprove our conclusions,” Zurek says.
Reference Activation of Semicore Electrons in Alkali Metals and Their Role in the B1–B2 Phase Transition under Pressure
Stefano Racioppi, Eva Zurek














Comments